Lake Charles company receives FDA Emergency Use Authorization for integrated gloves
Published 9:00 am Sunday, May 30, 2021
|
Getting your Trinity Audio player ready...
|
LAKE CHARLES /EINPresswire.com/ — Six months ago, when Jeremy Gormly, CRNA, walked out of the OR he told Mark Moore, “That’s the first time since coronavirus I’ve walked out of a surgery and didn’t feel dirty.” Moore nodded enthusiastically. Gormley had just used Moore’s newly designed ISOCUBE™, an added layer of protection to standard Personal Protective Equipment (PPE), for a surgical procedure.
Moore, a 36-year Certified Registered Nurse Anesthetist (CRNA), designed the negative pressure barrier device out of necessity to protect everyone in the OR including himself from airborne pathogens. This month, Moore’s firm, Prep Tech became the first company in the U.S. to receive an Emergency Use Authorization (EUA) from the Food & Drug Administration for two unique protective barrier enclosures.
Working in tandem with the U.S. Army since mid-2020, through both a license agreement and a Cooperative Research and Development Agreement (CRADA), Prep Tech has taken a leap forward in its mission to protect the surgical front line. Similar in scope to ISOCUBE™, the Army’s version, referred to as CAMIC (COVID-19 Airway Management Isolation Chamber), was developed for the same reasons. Using technology shared through the agreements, the Army tested and affirmed Prep Tech’s designs as being easy to use and viable for healthcare workers concerned with airborne contamination exposure.
According to Dr. Erich Wolf, M.D., Ph.D., a neurosurgeon and the co-founder and chief medical officer of Prep Tech, “The CAMIC license and CRADA between the Army and our firm allowed Prep Tech and Army inventors to co-develop the best possible devices to help protect health care workers and patients.”
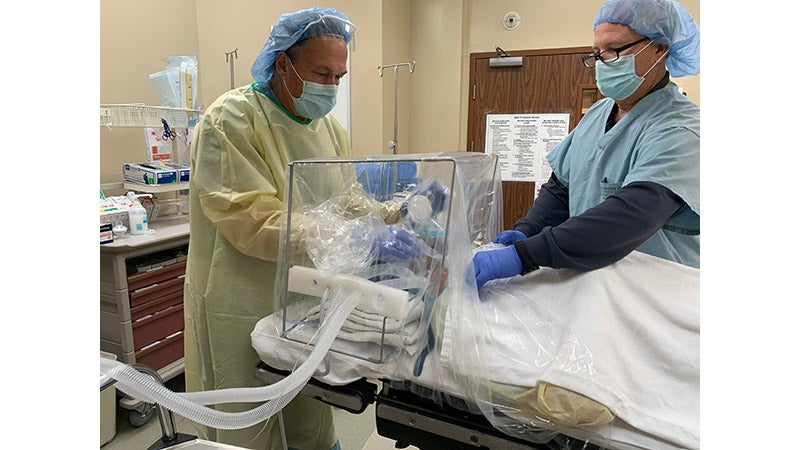
ISOCUBE™ SS is a multi-use stainless-steel base and rail system. ISOCUBE™ ONE is a single-use base and rail system. Both devices have a single-use, clear, negative pressure plastic-sheet chambers, and attaches to standard hospital or surgical beds, or stretchers, extending around the patient’s head, neck and shoulders. Four (4) access holes with integrated gloves, are built into the isolette chamber to allow for isolated patient access. The negative pressure environment is generated via wall-mounted hospital vacuum lines, and one or two negative pressure vacuums equipped with in-line HEPA filter(s).
Unlike the very few barrier enclosures available domestically or internationally, each patent-pending ISOCUBE™ model includes integrated gloves, a removable drape, and an integrated tubing portal for introducing standard surgical airway hoses and lines. Each ISOCUBE™ unit occupies a very small footprint in hospital surgical suite storage, and the units are economical enough to be purchased in multiples to be on-hand when a COVID-19 or suspected COVID-19 patient comes into the hospital.
“We envision ISOCUBE™ becoming a standard in hospitals globally as the world continues to face the spread of infectious diseases,” said Dr. Erich Wolf. “Prep tech has created two tools, available now, that surpass anything similar on the market”.
Visit www.preptechmed.com/isocube to learn more, order, and protect healthcare workers from continued pathogen exposure.
ABOUT PREP TECH
Prep Tech, LLC and its subsidiary Prep Tech Supplies, LLC are Louisiana-based medical
device companies founded by CEO Mark Moore, a certified registered nurse anesthetist, and Co-Founder and Medical Officer Dr. Erich Wolf, M.D., Ph.D., a biomedical engineer and neurosurgeon. Prep Tech’s mission is to develop innovative procedures and tools to improve the way healthcare professionals work inside and outside operating rooms. Existing innovations include its patented skin prep device, ULTRAPREP™, which increases OR efficiencies by 15-30% in certain extremity surgeries. Their headquarters is located in Lake Charles, Louisiana with operations in Lafayette, Louisiana and distributors nationwide. For more information visit preptechmed.com or contact Pete Prados, COO, via pete@preptechmed.com.
ABOUT ISOCUBE™ FDA EUA
Healthcare facilities using ISOCUBE™ must make available to patients and healthcare professionals, the accompanying Patient Fact Sheet and Healthcare Provider Fact Sheet, provided at pretechmed.com and on the FDA website. ISOCUBE™ has not been cleared or approved by FDA but has been authorized for emergency use by the FDA, under an EUA, for use by healthcare professionals during the COVID-19 pandemic, as an extra layer of barrier protection in addition to personal protective equipment (PPE). ISOCUBE™ is designed to prevent healthcare professionals’ exposure to pathogenic biological airborne particulates by providing temporary isolation of hospitalized patients with suspected or confirmed diagnoses of COVID-19 at the time of definitive airway management, when performing any airway-related medical procedures or during transport of such patients. The emergency use of the ISOCUBE™ has been authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices under section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.